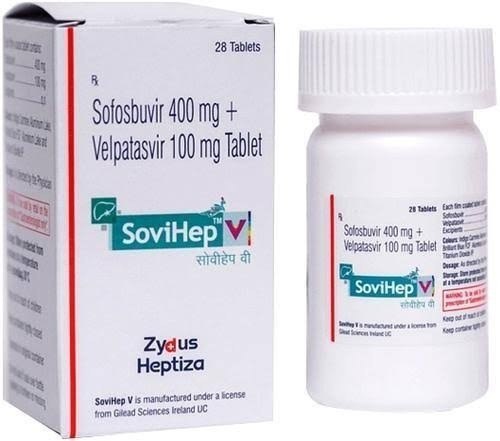
Sovihep D Sofosbuvir And Daclatasvir
Price 6500 INR/ Bottle
Sovihep D Sofosbuvir And Daclatasvir Specification
- Grade
- A
- Dosage Form
- tablet
- Molecular Formula
- Sofosbuvir (400mg) + Velpatasvir (100mg)
- Medicine Type
- Treatment of Chronic hepatitis C virus (HCV) infection
- Dosage
- 1 TABLET DAILY
Sovihep D Sofosbuvir And Daclatasvir Trade Information
- Minimum Order Quantity
- 1 Bottle
- Payment Terms
- Cash on Delivery (COD), Cash Advance (CA)
- Supply Ability
- 500 Bottles Per Day
- Delivery Time
- 2 Days
- Sample Policy
- Contact us for information regarding our sample policy
- Packaging Details
- 1*28
- Main Export Market(s)
- Asia
- Main Domestic Market
- All India
About Sovihep D Sofosbuvir And Daclatasvir


Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Sofosbuvir Tablets Category
Sofosbuvir 400Mg Velpatasvir 100Mg Tablets
Price 8000 INR / Bottle
Minimum Order Quantity : 1 Bottle
Dosage : 400 mg
Medicine Type : Tablet
Storage : 28 Tablets
Hepcvel Sofosbuvir And Velpatasvir Tablets
Price 9500.00 INR / Bottle
Minimum Order Quantity : 1 Bottle
Dosage : 1 TABLET DAILY
Medicine Type : Treatment of Chronic hepatitis C virus (HCV) infection
Grade : A
Storage : room temp
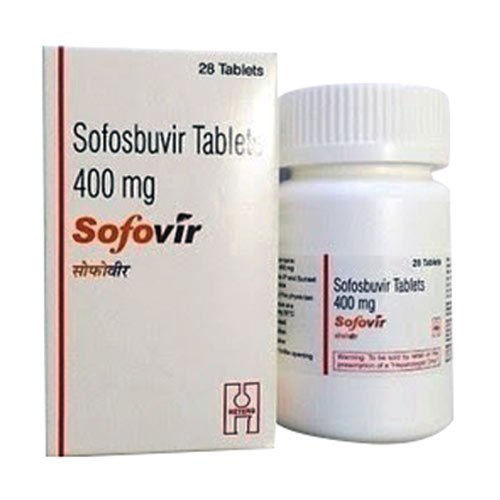
Sofovir 400 Mg Tablets
Price 3500 INR / Bottle
Minimum Order Quantity : 1 Bottle
Dosage : 1 TABLET DAILY
Medicine Type : In Chronic hepatitis C virus (HCV) infection
Grade : A
Storage : room temp
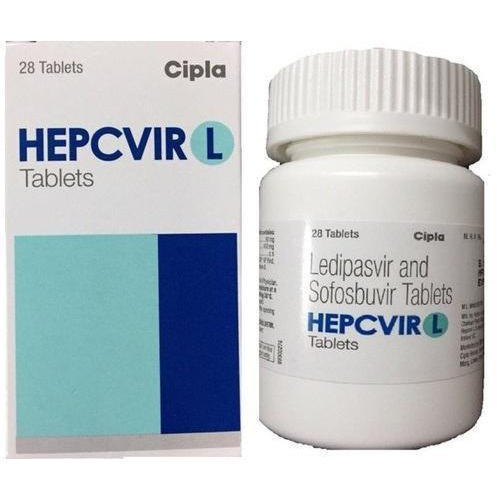
Hepcvir L Sofosbuvir And Ledipasvir Tablets
Price 9500.00 INR / Bottle
Minimum Order Quantity : 1 Bottle
Dosage : 1 TABLET DAILY
Medicine Type : Treatment of Chronic hepatitis C virus (HCV) infection
Grade : A
Storage : room temp
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry