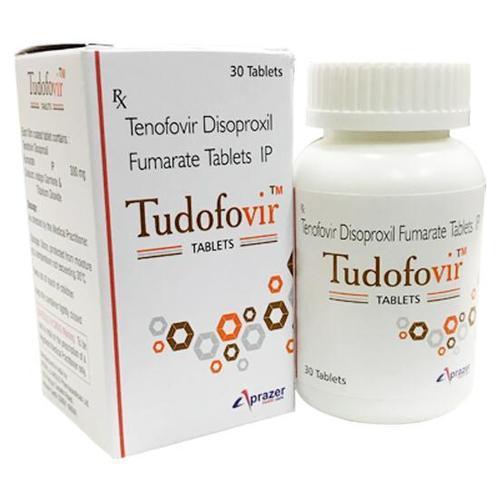
Tudofovir 300 Tablet
Price 650 INR/ Bottle
Tudofovir 300 Tablet Specification
- Drug Type
- Other Types
- Ingredients
- Each film coated tablet contains: Tenofovir Disoproxil Fumarate IP 300 mg Colours : Indigo Carmine & Titanium Dioxide. PHARMACOLOGY
- Physical Form
- Tablets
- Function
- Anti-Viral
- Recommended For
- HIV infection Chronic hepatitis B virus (HBV) infection
- Dosage
- 1 TABLETS DAILY
- Dosage Guidelines
- AS PER DIOCTOR ADVICE
- Suitable For
- Suitable For All
- Quantity
- 30 Boxes
Tudofovir 300 Tablet Trade Information
- Minimum Order Quantity
- 1 Bottle
- Payment Terms
- Cash on Delivery (COD), Cash Advance (CA)
- Supply Ability
- 500 Bottles Per Day
- Delivery Time
- 2 Days
- Sample Policy
- Contact us for information regarding our sample policy
- Packaging Details
- 1*30
- Main Export Market(s)
- Asia
- Main Domestic Market
- All India
About Tudofovir 300 Tablet
he pharmacokinetics of tenofovir disoproxil fumarate has been evaluated in healthy volunteers and HIV-1 infected individuals. Tenofovir pharmacokinetics is similar between these populations.
Absorption: Tenofovir disoproxil fumarate is a water soluble diester prodrug of the active ingredient tenofovir. The oral bioavailability of tenofovir from tenofovir disoproxil fumarate in fasted patients is approximately 25%. Following oral administration of a single dose of tenofovir disoproxil fumarate 300 mg to HIV-1 infected patients in the fasted state, maximum serum concentrations (C max ) are achieved in 1.0 0.4 hrs. C max and AUC values are 296 90 ng/mL and 2287 685 nghr/mL, respectively.
The pharmacokinetics of tenofovir are dose proportional over a tenofovir disoproxil fumarate dose range of 75 to 600 mg and are not affected by repeated dosing.
Effects of Food on Oral Absorption: Administration of tenofovir disoproxil fumarate following a high-fat meal (700 to 1000 kcal containing 40 to 50% fat) increases the oral bioavailability, with an increase in tenofovir AUC of approximately 40% and an increase in C max of approximately 14%. However, administration of tenofovir disoproxil fumarate with a light meal did not have a significant effect on the pharmacokinetics of tenofovir when compared to fasted administration of the drug. Food delays the time to tenofovir C max by approximately 1 hour.
Distribution: In vitro binding of tenofovir to human plasma or serum proteins is less than 0.7 and 7.2%, respectively, over the tenofovir concentration range 0.01 to 25 g/mL. The volume of distribution at steady state is 1.3 0.6 L/kg and 1.2 0.4 L/kg, following intravenous administration of tenofovir 1.0 mg/kg and 3.0 mg/kg.
Metabolism and Elimination: In vitro studies indicate that neither tenofovir disoproxil nor tenofovir are substrates of CYP450 enzymes.
Following IV administration of tenofovir, approximately 70-80% of the dose is recovered in the urine as unchanged tenofovir within 72 hours of dosing. Following single dose, oral administration of tenofovir disoproxil fumarate, the terminal elimination half-life of tenofovir is approximately 17 hours. After multiple oral doses of tenofovir disoproxil fumarate 300 mg once daily (under fed conditions), 32 10% of the administered dose is recovered in urine over 24 hours.
Tenofovir is eliminated by a combination of glomerular filtration and active tubular secretion.
INDICATIONS : TUDOFOVIR is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Tenofovir Tablets Category
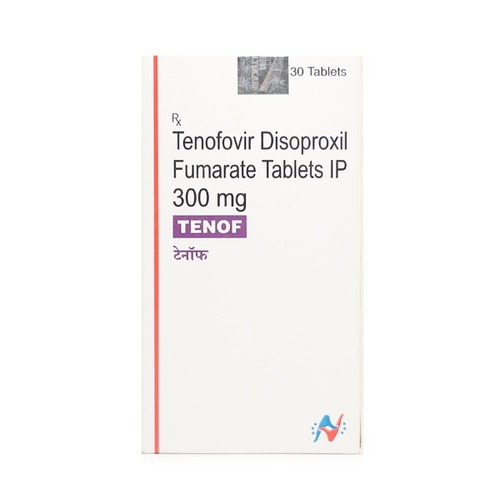
Tenof 300 mg Tablet
Price 600 INR / Bottle
Minimum Order Quantity : 1 Bottle
Storage Instructions : ROOM TEMPERATURE
Quantity : 30 Boxes
Ingredients : TENOFOVIR DISOPROXIL FUMRATE
Suitable For : Suitable For All
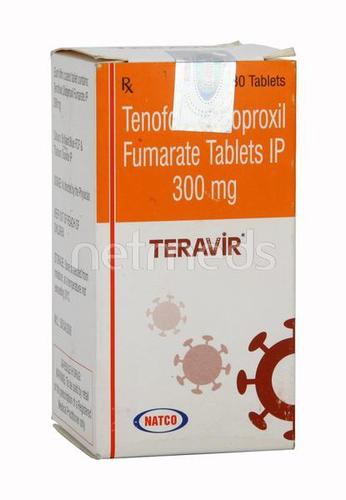
Teravir 300 mg Tablet
Price 750 INR / Bottle
Minimum Order Quantity : 1 Bottle
Storage Instructions : ROOM TEMPERATURE
Quantity : 30 Boxes
Ingredients : Tenofovir disoproxil fumarate (300mg)
Suitable For : Suitable For All
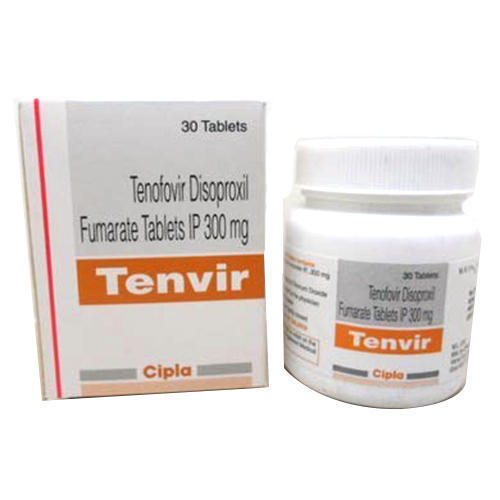
Tenvir 300MG
Price 950 INR / Bottle
Minimum Order Quantity : 1 Bottle
Storage Instructions : ROOM TEMPERATURE
Quantity : 1*30 TABLETS Boxes
Ingredients : TENEFOVIR 300 TABLETS
Suitable For : Suitable For All
Reviro 300mg Tablet
Price 750 INR / Bottle
Minimum Order Quantity : 1 Bottle
Storage Instructions : ROOM TEMPERATURE
Quantity : 30 Boxes
Ingredients : Tenofovir disoproxil fumarate (300mg)
Suitable For : Suitable For All
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry